- Statistically significant results with Quilience® (Mazindol ER) achieving a 7.1 point mean reduction in ESS from baseline compared to a 3.2 point reduction for placebo, p=0.0081
- Fast onset of action from the first week even at the low dose
- Safety and tolerability results consistent with prior mazindol clinical trials, no reported SAEs
- Favorable results on secondary endpoints including reduction in cataplexy attacks
ZURICH, SWITZERLAND / ACCESSWIRE / September 27, 2022 / NLS Pharmaceutics Ltd. (NASDAQ:NLSP)(NASDAQ:NLSPW) ("NLS" or the "Company"), a Swiss clinical-stage biopharmaceutical company focused on the discovery and development of innovative therapies for patients with rare and complex central nervous system disorders, announces positive top-line results from its Phase 2a clinical trial evaluating its lead product candidate, Quilience® (Mazindol ER), in the treatment of narcolepsy. The trial met its primary endpoint with high statistical significance.
Treatment with Quilience® 3mg per day (NLS-2) resulted in a 7.1 point mean reduction from baseline in excessive daytime sleepiness (EDS) over the 4-week treatment period based on the Epworth Sleepiness Scale (ESS), compared to a 3.2 point reduction for placebo (p=0.0081). The two treatment groups separated early and consistently throughout the study period confirming Mazindol ER's rapid onset of action and durable benefit.
Mean Change from Baseline in Epworth Sleepiness Scale (ESS)
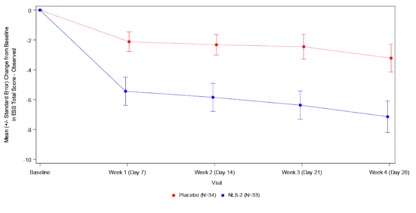
Total Score Observed by Visit; All Narcolepsy Types; Intent to Treat Patients
For the ESS score, the least squares (LS) means change from baseline to each visit and the standard error (SE) of Quilience® (NLS-2) versus placebo were all statistically significant at week 1 -4.3 (1.13) versus -1.1 (1.06) (p=0.0055), at week 2 -4.7 (1.14) versus -1.3 (1.06) (p=0.0035), at week 3 -5.0 (1.18) versus -1.6 (1.09) (p=0.0045), and at week 4 -5.8 (1.23) versus -2.1 (1.14) (-6.26, -1.15; p=0.0045).
The treatment arms were balanced in terms of patient demographics, baseline levels, and disease characteristics. Quilience® was well-tolerated and no safety concerns were identified. No serious adverse events (SAEs) were reported. With regard to the reduction in cataplexy attacks in narcolepsy Type 1 patients (NT1), a key secondary endpoint, Quilience® consistently outperformed placebo at all time points. In the trial's open label extension (OLE) study, 85% of patients including those with NT1, elected to rollover and receive Quilience® for up to 6 months as a monotherapy. NLS plans to release interim results from the OLE study before year-end and announce final results in the first quarter of 2023.
"These results demonstrate Mazindol ER's rapid onset of action and ability to significantly improve wakefulness in patients with moderate to severe narcolepsy," said Bruce Corser, M.D., Medical Director of the Sleep Management Institute in Cincinnati. "With the strong signal in reducing the number of cataplexy attacks in patients with NT1, Quilience® has promise to become a much-needed alternative for patients who have not achieved adequate symptom relief from currently available treatments."
"These results reinforce the significant efficacy seen in our interim analysis earlier this year and confirm Mazindol ER's safety and tolerability profile established in over 40 years of on-label and off-label use," said George Apostol, M.D. MS, Chief Medical Officer and Global Head of R&D at NLS. "We plan to present the final results from this Phase 2a clinical trial at a future scientific meeting, and look forward to meeting with the U.S. Food and Drug Administration (FDA) later this year to discuss the design of a pivotal clinical program for Quilience® as we focus on bringing this important solution to patients."
NLS will host a Key Opinion Leader (KOL) Event on September 30, 2022 to review the top-line Phase 2a results. The KOL Event will also feature study investigators and an independent practitioner that will discuss the clinical results and offer perspectives on the potential for Quilience to treat patients with both NT1 and narcolepsy type 2 (NT2). A Question-and-Answer session will follow the presentation of the results and KOL discussion.
Details for the NLS Pharmaceutics Quilience® Phase 2a KOL Event:
Date/Time: September 30, 2022; 10:00am to 11:30am Eastern Time
Registration: Please use the following link to both register and access the event at the designated date and time: https://wsw.com/webcast/cc/nlsp/1390158
Moderator: George Apostol, M.D., Chief Medical Officer & Global Head of R&D, NLS Pharmaceutics
Additional Speakers:
Bruce Corser, M.D., Medical Director, Sleep Management Institute, Cincinnati, OH: Pulmonary and Sleep Medicine Specialist
Anne Marie Morse, D.O., Geisinger Medical Center, Danville, PA: Specialties in Pediatric Neurology and Sleep Medicine
Thomas Stern, M.D., Novant Health, Huntsville, NC: Advanced Respiratory and Sleep Medicine Specialist
"These significant results demonstrate the potential for our proprietary Mazindol ER formulation to become the therapy of choice for narcolepsy and other related sleep-wake disorders," said Alex Zwyer, Chief Executive Officer of NLS Pharmaceutics. "I am proud of our ability to execute effectively on this trial, having recruited patients in a quick time frame and exceeding our enrollment target by 12%. With so many patients expressing dissatisfaction with their narcolepsy treatment regimen, we remain dedicated to advancing this differentiated product candidate, which has now shown the ability to treat the core symptoms of the disorder as a monotherapy with once-daily oral dosing."
About the Phase 2a trial (NLS-1021)
NLS-1021 is a multi-center, randomized, prospective Phase 2a clinical trial evaluating Quilience® (Mazindol ER) as a once-daily monotherapy for the treatment of EDS and cataplexy, the primary symptoms of narcolepsy. The trial was designed to enroll 60 patients diagnosed with NT1 or NT2 to receive treatment with either Quilience® up to 3mg once daily for 28 days, or placebo. A total of 67 patients were enrolled and randomized 1:1 into each treatment arm. The primary endpoint of the trial is the change from baseline in EDS as measured by the ESS, and a key secondary endpoint is the change from baseline in mean weekly number of cataplexy attacks in the subset of patients with cataplexy. Patients completing the 4-week clinical study period were eligible to enroll in an open label extension study (NLS-1022), where each patient was given the option to receive Quilience® monotherapy for up to an additional 6 months.
About NLS Pharmaceutics Ltd.
NLS Pharmaceutics Ltd. is a Swiss clinical-stage biopharmaceutical company led by an experienced management team with a track record of developing and repurposing product candidates to treat rare and complex central nervous system disorders. The Company's lead product candidate, Quilience®, is a proprietary extended-release formulation of Mazindol (Mazindol ER) and is being developed for the treatment of narcolepsy, and potentially other sleep-wake disorders such as idiopathic hypersomnia (IH), for which NLS recently obtained Orphan Disease Designation (ODD) from the European Medicines Agency (EMA). Mazindol is a triple monoamine reuptake inhibitor and partial Orexin-2 Receptor agonist, which was used for many years to treat patients diagnosed with narcolepsy in compassionate use programs. A Phase 2a multi-center U.S. clinical trial evaluating Quilience® in adult subjects suffering from narcolepsy met its primary endpoint with high statistical significance and demonstrated a favorable safety and tolerability profile. NLS also successfully completed a Phase 2 study in the U.S. evaluating Nolazol® (Mazindol Controlled-Release) in adult subjects suffering from ADHD. The study met all primary and secondary endpoints and Nolazol® was well-tolerated. Quilience® has received Orphan Drug Designations both in the U.S. and in Europe for the treatment of narcolepsy. Up to 1/3 of narcoleptic patients are also diagnosed with ADHD.
Safe Harbor Statement
This press release contains expressed or implied forward-looking statements pursuant to U.S. Federal securities laws. For example, NLS is using forward-looking statements when it discusses the ability of Quilience® to treat ESS and cataplexy in patients with narcolepsy, the timing of the release of interim and final results from the OLE study, the timing of the presentation of final results from the Phase 2a clinical trial and the timing of its expected meeting with the FDA.These forward-looking statements and their implications are based on the current expectations of the management of NLS only and are subject to a number of factors and uncertainties that could cause actual results to differ materially from those described in the forward-looking statements. The following factors, among others, could cause actual results to differ materially from those described in the forward-looking statements: changes in technology and market requirements; NLS may encounter delays or obstacles in launching and/or successfully completing its clinical trials; NLS' products may not be approved by regulatory agencies, NLS' technology may not be validated as it progresses further and its methods may not be accepted by the scientific community; NLS may be unable to retain or attract key employees whose knowledge is essential to the development of its products; unforeseen scientific difficulties may develop with NLS' process; NLS' products may wind up being more expensive than it anticipates; results in the laboratory may not translate to equally good results in real clinical settings; results of preclinical studies may not correlate with the results of human clinical trials; NLS' patents may not be sufficient; NLS' products may harm recipients; changes in legislation may adversely impact NLS; inability to timely develop and introduce new technologies, products and applications; loss of market share and pressure on pricing resulting from competition, which could cause the actual results or performance of NLS to differ materially from those contemplated in such forward-looking statements. Except as otherwise required by law, NLS undertakes no obligation to publicly release any revisions to these forward-looking statements to reflect events or circumstances after the date hereof or to reflect the occurrence of unanticipated events. More detailed information about the risks and uncertainties affecting NLS is contained under the heading "Risk Factors" in NLS' annual report on Form 20-F for the year ended December 31, 2021 filed with the Securities and Exchange Commission (SEC), which is available on the SEC's website, www.sec.gov, and in subsequent filings made by NLS with the SEC.
Corporate Contact
Alex Zwyer, CEO: +41 44 512 21 50
Investor Relations Contact
Cindy Rizzo: +1 908-229-7050
Media Contact
Pascal Nigen: +1 917-385-2160
Alpha Bronze, LLC
www.nlspharmaceutics.com
SOURCE: NLS Pharmaceutics Ltd.
View source version on accesswire.com:
https://www.accesswire.com/717598/NLS-Pharmaceutics-Announces-Positive-Top-Line-Results-from-its-Phase-2a-Clinical-Trial-of-Once-Daily-QuilienceR-Mazindol-ER-for-the-Treatment-of-Excessive-Daytime-Sleepiness-and-Cataplexy-in-Patients-with-Narcolepsy













