Study will evaluate patients treated for cervical degenerative disc disease at two contiguous vertebral levels with the M6-C disc compared to ACDF
Orthofix Medical Inc. (NASDAQ:OFIX), a global medical device company with a spine and orthopedics focus, today announced the first patient implant in a U.S. Food and Drug Administration (FDA) clinical study that will evaluate the safety and effectiveness of the M6-C™ artificial cervical disc compared to anterior cervical discectomy and fusion (ACDF) for the treatment of contiguous two-level symptomatic cervical radiculopathy.
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20210802005105/en/
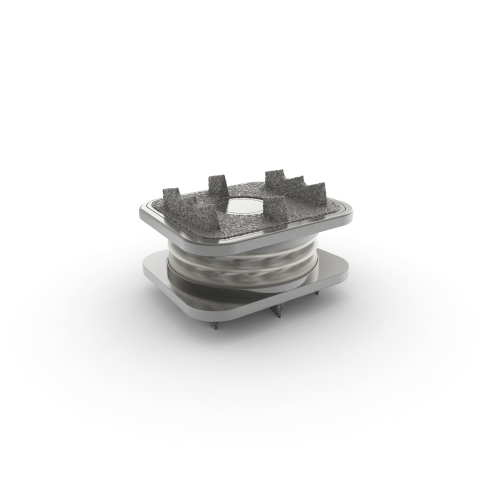
Image of the Orthofix M6-C™ artificial cervical disc (Photo: Business Wire)
“Artificial cervical disc replacement is rapidly becoming the standard of care for patients suffering from degenerative cervical disc disease because it preserves motion, unlike ACDF procedures,” said Dr. Todd Lanman, a spinal neurosurgeon with Beverly Hills-based Lanman Spinal Neurosurgery and founder of the national ADR Advanced Disc Replacement Spinal Restoration Center who performed the first patient implant in the study. “The M6-C artificial cervical disc two-level study will provide additional data to validate the effectiveness of disc replacement over fusion in patients suffering from degeneration in two contiguous levels.”
Being conducted under a U.S. Investigational Device Exemption (IDE), the study will evaluate the safety and effectiveness of the M6-C artificial cervical disc compared to ACDF for the treatment of contiguous two-level symptomatic cervical radiculopathy at vertebral levels from C3 to C7 with or without spinal cord compression.
Patients will be concurrently enrolled in the M6-C treatment group and the ACDF control group. For the study, 263 patients will undergo either a two-level cervical artificial disc procedure or an instrumented ACDF procedure as per site group assignment. Patients will be evaluated clinically, radiographically, and via the collection of patient-reported outcomes at six weeks, three months, six months, 12 months and 24 months. The primary endpoint is overall success at 24 months.
“To date there have been more than 55,000 implantations worldwide of the M6-C artificial cervical disc,” said Orthofix President of Global Spine Kevin Kenny. “We are excited to begin this study as it supports our commitment to continue to build upon the global body of evidence supporting the use of the M6-C artificial cervical disc as a safe and effective treatment for patients suffering from the debilitating pain of degenerative disc disease.”
About the M6-C Artificial Cervical Disc
The M6-C artificial cervical disc was FDA-approved for single-level implantation from C3 to C7 in 2019. Designed to restore physiologic motion to the spine, the M6-C disc is indicated as an alternative to cervical fusion. The M6-C disc preserves motion by restoring biomechanical function at the treated level after native disc removal and potentially reduces subsequent degeneration of adjacent vertebral segments. The M6-C device is the only artificial cervical disc that mimics the anatomic structure of a natural disc by incorporating an artificial visco-elastic nucleus and fiber annulus into its design. Like a natural disc, this unique construct allows for shock absorption at the implanted level, as well as provides a controlled range of motion when the spine transitions in its combined complex movements.
About Orthofix
Orthofix Medical Inc. is a global medical device company with a spine and orthopedics focus. The Company’s mission is to deliver innovative, quality-driven solutions as we partner with health care professionals to improve patient mobility. Headquartered in Lewisville, Texas, Orthofix’s spine and orthopedics products are distributed in more than 60 countries via the Company’s sales representatives and distributors. For more information, please visit www.Orthofix.com.
Forward-Looking Statements
This communication contains forward-looking statements within the meaning of Section 21E of the Securities Exchange Act of 1934, as amended, and Section 27A of the Securities Act of 1933, as amended, relating to our business and financial outlook, which are based on our current beliefs, assumptions, expectations, estimates, forecasts and projections. In some cases, you can identify forward-looking statements by terminology such as “may,” “will,” “should,” “expects,” “plans,” “anticipates,” “believes,” “estimates,” “projects,” “intends,” “predicts,” “potential,” or “continue” or other comparable terminology. These forward-looking statements are not guarantees of our future performance and involve risks, uncertainties, estimates and assumptions that are difficult to predict, including the risks described in Part I, Item 1A under the heading Risk Factors in our Annual Report on Form 10-K for the year ended December 31, 2020 (the “2020 Form 10-K”). In addition to the risks described there, factors that could cause or contribute to such differences may include, but are not limited to: the risk that FDA approvals may be delayed or not be obtained; the risk that surgeons may be slow to adopt the M6-C artificial cervical disc; the risk that two-level study or clinical experience and data may indicate that treatment with the M6-C cervical disc for two-level symptomatic cervical radiculopathy does not improve patient outcomes as much as previously believed, or otherwise call into question the benefits of its use to patients, hospitals and surgeons; the risk that the product may not perform as intended and may therefore not achieve commercial success; the risk that competitors may develop superior products or may have a greater market position enabling more successful commercialization; the risk that insurance payers may decline to reimburse healthcare providers for the use of our products.
This list of risks, uncertainties and other factors is not complete. We discuss some of these matters more fully, as well as certain risk factors that could affect our business, financial condition, results of operations, and prospects, in reports we file from time-to-time with the SEC, which are available to read at www.sec.gov. Any or all forward-looking statements that we make may turn out to be wrong (due to inaccurate assumptions that we make or otherwise), and our actual outcomes and results may differ materially from those expressed in these forward-looking statements. You should not place undue reliance on any of these forward-looking statements. Further, any forward-looking statement speaks only as of the date hereof, unless it is specifically otherwise stated to be made as of a different date. We undertake no obligation to update, and expressly disclaim any duty to update, our forward-looking statements, whether as a result of circumstances or events that arise after the date hereof, new information, or otherwise.
View source version on businesswire.com: https://www.businesswire.com/news/home/20210802005105/en/
Contacts
Alexa Huerta
Investor Relations
Tel 214 937 3190
alexahuerta@orthofix.com
Denise Landry
Media Relations
Tel 214 937 2529
deniselandry@orthofix.com














